Module 3 – Radiotherapy in Practice
Learning
objectives
·
Learn the
basic principles of how radiotherapy works
·
Understand
the different types of radiotherapy treatments available
·
Learn about
the process of radiotherapy treatment preparation and delivery
·
Recognise
possible short term and long term side effects of radiotherapy
Introduction
You may now
have much experience of radiotherapy but here are some things to consider
·
Radiotherapy
is an important anti-cancer therapy, and is used in the treatment of 40% of
patients who are cured of cancer (compared to 2% for chemotherapy). 50% of
cancer patients will need radiotherapy at some point in their cancer journey
·
Modern
radiotherapy makes extensive use of imaging, computing, and engineering to
direct radiation to the tumour target with high precision
·
Radiotherapy
is an extremely cost effective cancer treatment. A course of radiotherapy costs
about £2500, compared to £5500 for a surgical procedure and £13500 for a course
of chemotherapy
A brief
glossary
Teletherapy / External Beam radiotherapy – This means the radiation source is outside
and at some distance from the patient
Brachytherapy
- this means the radiation source is inside or on the surface of the patient
Radionuclide therapy – a radioactive isotope is injected or
ingested by the patient
X-ray – electrically generated ionising radiation.
We use high energy x-rays for most of our radiotherapy treatments
Gamma ray – naturally occurring ionizing radiation
from decay of radioactive isotopes. We use radioactive sources in brachytherapy
and implants which emit gamma rays. Physically speaking there is no difference
between a gamma ray and an x-ray of the same energy.
X-ray energy – we express x-ray energies as the voltage
of the accelerating field used to generate the x-rays. This is typically
kilovolts to megavolts.
How does
radiotherapy work?
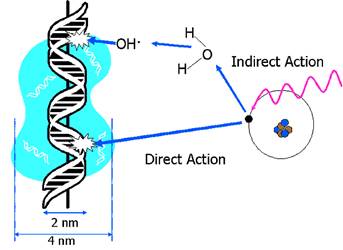
Radiotherapy
works by damaging DNA. High energy x-rays interact with matter and produce high
energy electrons. It is these electrons that damage DNA in one of two ways
|
The direct effect Electrons
hit the DNA backbone and induce single strand breaks and double strand
breaks. |
|
The indirect effect Electrons
interact with a water molecule close to the DNA backbone. The water molecule
is split into reactive H· and OH · radicals and these induce double and
single stranded breaks |
We are
mainly interested in double stranded DNA breaks as these cause the most
difficulty to the cell. Healthy cells have functional DNA repair mechanisms and
will either repair DNA damage or recognize the genotoxic injury and undergo
apoptosis. Tumour cells have dysregulated DNA repair and replication pathways
and will therefore undergo mitotic cell death in response to radiotherapy.
So without
matter to interact with, an x-ray won’t deposit radiation dose (a bit like that
philosophical question about a tree in a forest falling down when no-one is
around – does it bother to make a sound). This sounds a bit esoteric but is
actually really useful in the build-up
effect.
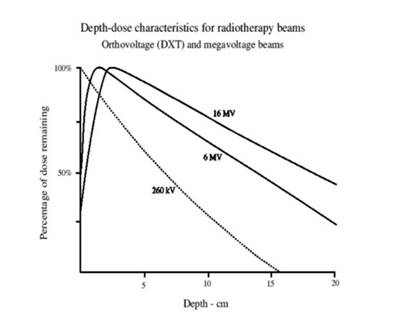
This graph
shows the amount of energy deposited by an x-ray as it passes through tissue,
and its relationship with its energy. The key thing to notice here is that the
higher energy x-rays don’t deposit their maximum energy at the skin, instead
they deposit maximum energy at some depth into the patient. This is because the
x-rays have so much energy they don’t really start interacting with tissue
until they are further in to the body. This means that we can give a higher
dose to a deep seated tumour than to the skin on the surface of the patient.
When radiotherapy first started being used, the x-rays were of such low energy
that most of the radiation dose went into the skin causing radiation burns.
Radiation
dose is measured in Gray. It is the SI unit of absorbed radiation dose and is
equivalent to 1 joule of energy per kilogram of tissue. It’s about the amount
of thermal energy in a cup of coffee. Normally we aim to deliver about 60Gy of
radiation for a curative treatment.
Modern
radiotherapy tries to deliver as high a dose as possible to the tumour, whilst
minimizing the dose to healthy surrounding tissues. There are several ways we
can achieve this goal:
·
Shape the
radiation dose as tightly as possible to the shape of the tumour
·
Break up the
treatment into smaller daily treatments (fractions) to allow health normal
tissues to repair between treatments
·
Enhance
tumour cell kill by combining drug therapy with radiation therapy
(chemo-radiotherapy)
Conformal
radiotherapy. Shaping the radiation to the target.
Radiotherapy
technology has advanced considerably in the last 10-15 years, allowing us to
shape radiation dose with greater accuracy. Modern radiotherapy typically uses
multiple beams which treat the target from different directions. The dose in
the entry path and exit path of the beam is low, compared to the dose in the
area of overlap of the beams.
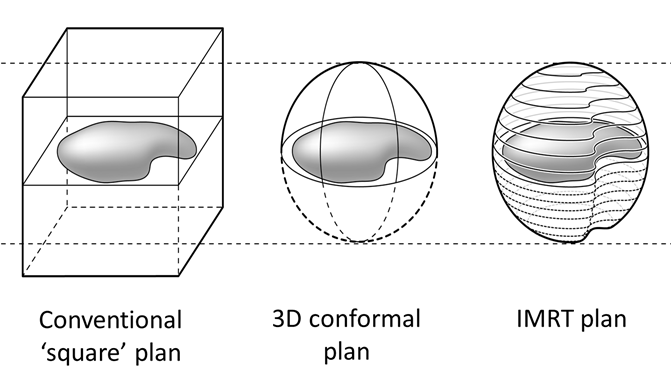
3D conformal
radiotherapy means that the radiation beams are shaped to match the profile of
the tumour. This is achieved by a device called a multileaf collimator. It
moves leaves of lead into the beam go give it the correct shape and shield
healthy tissues around the tumour target as much as possible. This is easier said than done – for
a 6 million electron volt machine, the leaves of lead have to be 10cm think,
and fit together perfectly to that only a minimal amount of radiation leaks out
in between the leaves.

Intensity
modulated radiotherapy is the next step in conformal radiotherapy. There the
machine moves the MLC leaves during treatment to build a very complex
distribution of dose. Often the machine will rotate around the patient as the
beam shape is being changed. It’s much
easier to visualise with a movie than in words, so check this excellent youtube video showing an animation of a rotational IMRT
system.
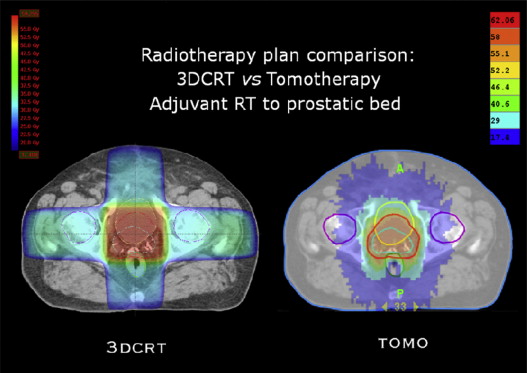
If you look
at this dose distribution, you can see the difference that can be achieved
between 3D conformal radiotherapy and rotational IMRT (tomotherapy) plans. The red line is the
tumour target (in this case the prostate gland), and you can see in the colour
wash the dose distribution, with high dose in red and low dose in blue. Note
how the high dose is shaped to match the shape of the target with the IMRT
plan.
You may hear
about a range of treatment machines which all deliver highly conformal
radiotherapy in essentially the same way – by breaking the radiation beam up
into lots of tiny beamlets which can be turned on or off individually, and
rotating the x-ray beam around the patient. Where the beams converge, the
radiation dose will accumulate.
|
|
|
|
|
The TomoTherapy
unit has geometry like that of a traditional CT scanner. The radiation beam
can rotate fully round the patient as they move through the machine |
VMAT or volumetric modulated arc
therapy, uses a traditional linear accelerator gantry rotating round the patient. |
Cyberknife uses a compact linear
accelerator mounted on the end of a 5 axis robotic arm. It can move and
arcround the patient with a high number of degrees of freedom |
There is a
cost to using these types of highly conformal radiotherapy. You can’t beat the
laws of physics and for each little beamlet there will be an entry dose and an
exit dose as the x-ray beam passes through the patient. As a result IMRT tends
to smear low dose through a larger volume of the patient. This may be
particularly relevant in children, where the low dose bath increases the risk of growth effects and of second
tumour formation.
As an aside,
it’s worth remembering that low dose radiation is more mutagenic than high dose
radiation. High dose radiation will either kill cells, or induce repair
mechanisms that fix DNA damage. Low dose radiation may induce low level DNA
damage that is not detected and repaired, leading to subsequent mutation.
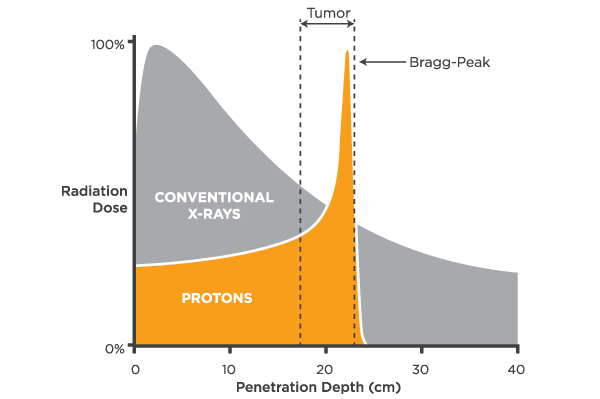
Proton beam
therapy is a novel form of radiation therapy that uses particle beams instead
of x-rays. The advantage of a particle beam is that the particle can be tuned
to stop at a certain distance in the patient. As a result, instead of having a
low exit dose, like x-rays, proton beam therapy can achieve no exit dose.
Proton beam therapy will start in the UK in 2018, and is likely to focus on
children’s tumours in the first instance.
Fractionation
– breaking radiotherapy up into multiple daily treatments
By breaking
treatment up into small daily treatments, we see a cell killing effect in the
tumour cells (cell loss), depending on the speed with which they regrow
(repopulate) between treatments. In contrast, normal tissues won’t regrow much
at all, but they will repair most of the DNA damage between treatments. The
half-life for mammalian DNA repair mechanisms is about 8 hours, and a 24 hour
break between treatments allows 3 half-lives worth of repair to occur. This is
why most radiotherapy is given as a 6-7 week course of daily radiotherapy
treatment fractions. Of course tumours
don’t grow at weekends so we don’t need to treat them on a Saturday or Sunday!
Sometimes we
use the fractionation effect in reverse. We deliver a single large dose of
radiotherapy to completely ablate everything within the treatment volume. This
is known as radiosurgery. In order to achieve this, we have to ensure that we
deliver minimal dose to the surrounding tissues. In the brain, we often do this
by immobilising the patient using a stereotactic frame, similar to that used
for neurosurgery.
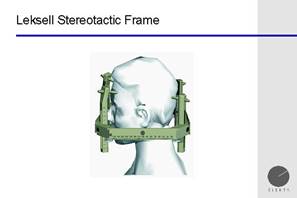
Thus the treatment
is often known as stereotactic radiosurgery or SRS. Note that although most SRS
is undertaken for intracranial lesions such as brain metastases, vestibular
schwannoma and arterio-venous malformations, stereotactic radiosurgery can be
undertaken in the body too, particularly lung, liver and bone lesions.
Chemo-radiation
– enhancing tumour cell kill
In some
situations, we combine low dose chemotherapy with radiotherapy to enhance cell
kill. When chemotherapy is given in this way it is said to be a
radiosensitiser. The results can be quite dramatic. Chemo-radiation in stage 3
cervix cancer yields dramatic improvements in survival:
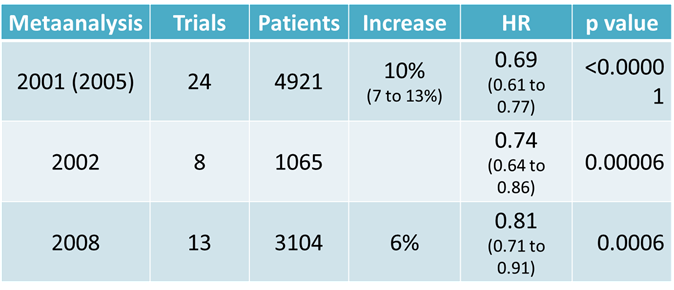
However,
chemoradiation also has an increased effect on healthy tissues. Patients in
these cohorts had an increased risk of complications such as vesico-vaginal
fistula, rectal ulceration and pelvic fractures.
Clinical
contexts for radiation therapy
There are a
number of ways in which we can use radiotherapy as an anti-cancer treatment
·
Primary
curative treatment. This is
where radiotherapy is used in place of surgery as the main modality for cancer
cure. Often it is the case that surgery would involve removal of the organ
bearing the cancer, resulting in significant loss of function for the patient.
Radiotherapy allows curative treatment to be delivered without removing the
organ, and is often therefore an organ preserving treatment. Good examples of
this are larynx cancer, which would involved removal of the larynx if treated
surgically, and anal cancer, which would involve a large AP resection and stoma
if treated surgically. Some examples of primary therapy include
o
Skin cancers
o
Cervix
cancer
o
Head &
neck tumours
o
Lung cancer
o
Anal cancer
o
Lymphoma
o
Prostate
cancer
The results can be very impressive. This is
an example of a nasty squamous cell carcinoma on the temple, that would have
needed extensive resection and grafting. After treatment the skin is thing and
there is telangiectasia visible but a good cosmetic result has been achieved.
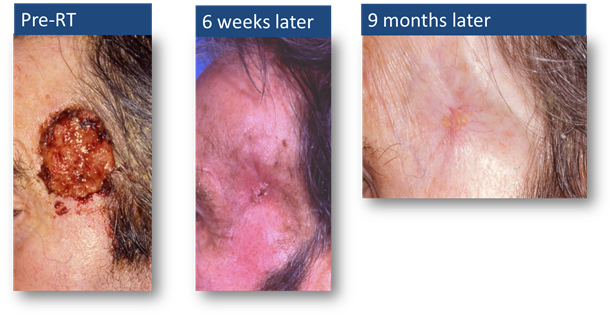
This is a patient with a bulky cervical
cancer, treated with radiotherapy. Fast growing squamous cancer can often
respond rapidly to radiotherapy.
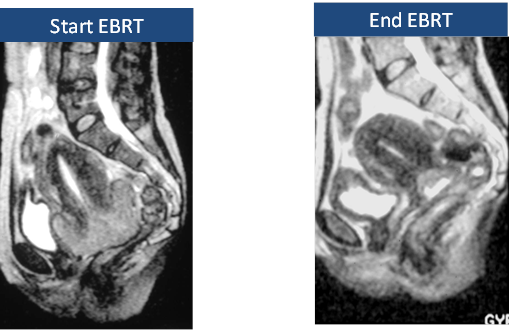
·
Adjuvant
radiotherapy. This is
treatment given after surgical resection to control any microscopic disease
that may have been left behind, either in the site of the tumour or adjacent
lymph nodes. Radiotherapy is given to improve local control, and it has been
well demonstrated that improved local control of early stage cancer leads to
improved survival. This represents a large volume of what we do in radiotherapy
and example include:
o
Breast
cancer
o
Rectal
cancer
o
Endomterial
sarcoma
o
Brain
tumours
o
Sarcoma
·
Palliative
radiotherapy. This is
treatment given to patients with advanced disease to improve symptoms or
maintain function. As patients liver longer alongside metastatic disease we are
giving more palliative radiotherapy. It is useful for localised symptoms such
as bone pain, nerve compression pain, and low volume bleeding from a tumour
surface (radiotherapy won’t work if the bleeding is coming from a large
vessel).
Clinical
pathway for radiation therapy
All patients
receiving radiotherapy treatment follow a pathway for preparation of their
radiotherapy, shown in the diagram below:
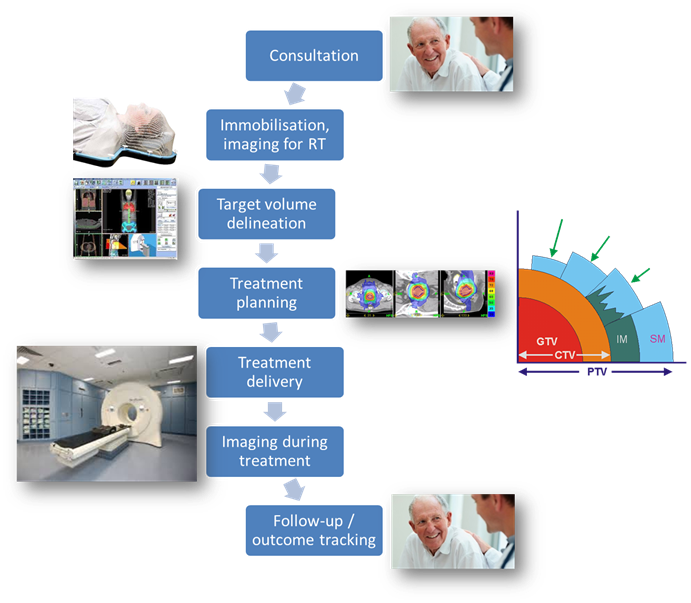
The key
things we want to achieve during radiotherapy are
·
Inform the
patient what to expect. They are likely to tolerate treatment and maintain a
stable treatment position if they know exactly what is happening.
·
Find a way
to minimize motion of the target area during radiotherapy. This can be done by
ensuring the patient if in a comfortable, stable and reproducible position each
time they come for radiotherapy.
·
Calculate a
radiotherapy plan that delivers maximal dose to the tumour, and minimal dose to
surrounding healthy tissues.
·
Use imaging
techniques on the treatment machine to ensure that we are treating what we are
supposed to be treating.
·
Monitor the
patient during therapy for side effects of treatment.
You’ll see
more about what happens to a patient on the radiotherapy tour. One of the cool
things we do to keep patients in the right position is to make thermoplastic
shells. These are made of a special plastic sheeting which is flexible when
warmed in a water bath to 40 degrees, and then set at room temperature. Again
it is much easier to watch than explain, so here is a short video of Sarah
Knight, one of our radiographers, making a thermoplastic shell for brain radiotherapy.
Side effects
of radiotherapy
As they say
on Star Trek, you can’t change the laws of physics, and thus if we are treating
a tumour within the body with radiation, there has to be some exposure of
adjacent structures to radiation, which will cause unwanted side effects. We
think of side effects as acute effects which occur during therapy and last for
a few weeks afterwards, and late side effects, which can come on years to
decades after radiotherapy.
·
Acute
toxicity starts about
2 weeks after the beginning of radiotherapy, and tends to affect the fastest
proliferating tissues, causing dermatitis, stomatitis and enteritis. This is
what causes the nausea and vomiting after acute radiation exposure. Hair loss
can also occur in the area where the radiation fields hit the skin, typically
2-3 weeks after the start of radiotherapy. Radiotherapy also causes fatigue, no
matter which part of the body is being treated, presumably as part of the
response to radiation injury.
·
Late
toxicity is typically
caused by vascular injury to normal tissues after radiotherapy. Radiation
effectively causes a small vessel obliterative endarteritis, and fibrosis
occurs in response to the ischaemia and cytokine release associated with this.
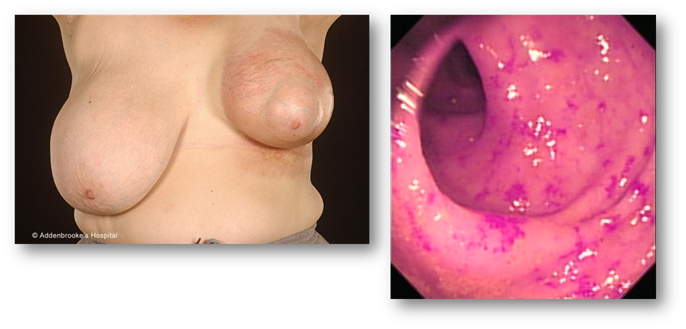
These images show late effects of
radiotherapy. On the left is a poor cosmetic outcome from radiotherapy to the
left breast. You can see evidence of breast shrinkage as a result of fibrosis,
and extensive skin telangiectasia in the radiation fields. The fibrosis can
also be painful. On the right you can see evidence of small vessel damage in
the rectum following pelvic radiotherapy.
·
Growth and
risk of second malignancy. When we
use radiotherapy in children we worry about the effect of radiation on growth.
Radiation to the epiphyses of long bones will result in premature fusion and
loss of stature. Asymmetric radiation to the spine will result in
scoliosis. Radiation itself can also
cause malignancy, and the risk of this varies depending on the volume and type
of normal tissue being treated. A good rule of thumb is that for most curative
treatments the risk of second malignancy is approximately 3% per decade of life
after radiotherapy for children and 1% per decade of life for adults.
Conclusion
Radiotherapy
is essentially a spatially targeted anti-cancer therapy which induces DNA
damage in cells. The damage is lethal to tumour cells, but can be repaired or
recognized in healthy tissues.
Radiotherapy
is a tool for achieving local tumour control. It can be used in place of
surgery, or as an adjunct to surgery.
New
technologies in radiation therapy allow increased precision of the delivery of
radiation dose, and image-guided radiotherapy uses a range of imaging technique
to ensure we deliver treatment to the target.
Nonetheless,
radiotherapy has important acute and long term side effects and has to be used
with caution.
For more
details, come to the tour of the radiotherapy department which is usually on
the second Monday of your attachment, or take a look at extension resource E2
– a virtual tour of the radiotherapy pathway.